Anti-HIV therapy: IgG3 is the new kid on the block !
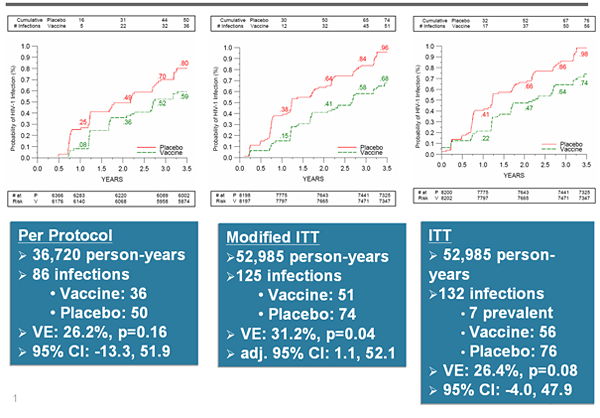
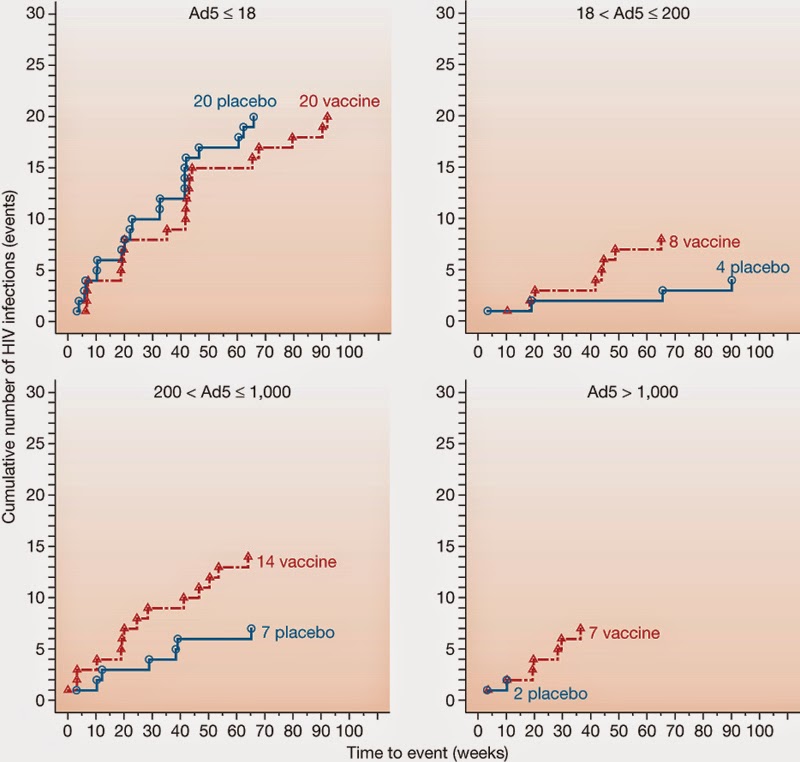
In a fantastic new paper by Chung et al., (2014) that accompanied the paper described in my previous Blog by Yates et al., (2014), the role played by IgG3 has been described very elegantly in the context of 2 HIV vaccines-RV144 and VAX003. Chung et al., show how IgG3 might be able to fight HIV infection in the absence of viral neutralization. Background: RV144: ALVAC-HIVvCP1521 prime and recombinant gp120 AIDSVAX B/E boose VAX003: AIDSVAX B/E bivalent rgp120 given with alum in 7 doses Results: * RV144 elicited a polyfunctional antibody response associated with the selective induction of IgG3 antibodies. * VAX003 elicited a monofunctional antibody response characterized by selection of the functionally inert IgG4 antibody subclass, likely due to repeated administration of gp120 protein vaccination which decreased IgG3 subclass levels. *RV144 induced both IgG1 and IgG3 antibodies targeting the V2 crown,...