STEP Trial: A vaccine that surprised many with increased risk of disease in vaccinees
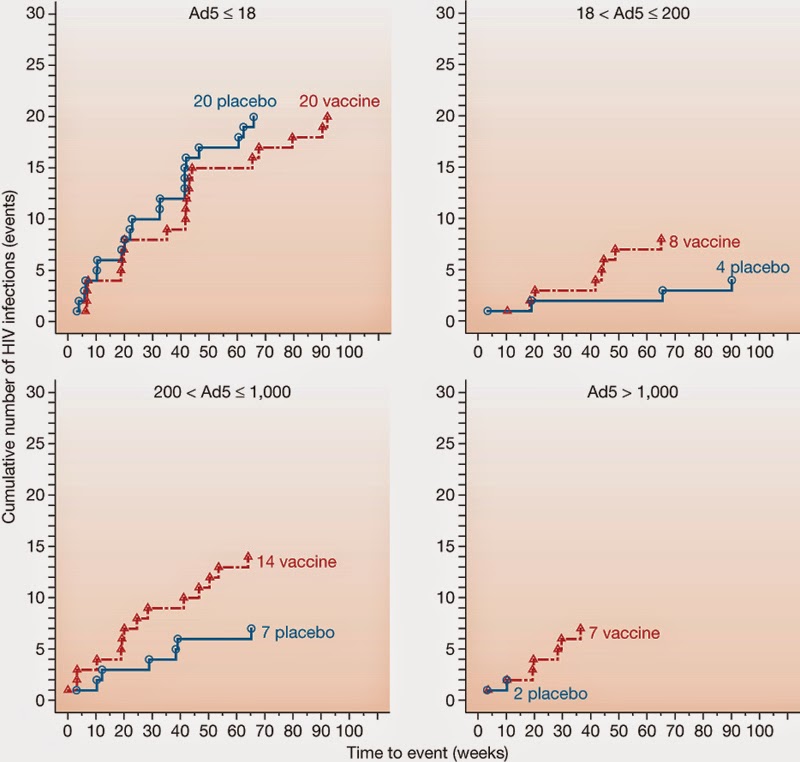
HVTN/STEP Trial: A vaccine that surprised many with increased risk of disease in vaccinees: A Phase IIb ‘test of concept’ trial of Merck’s MRKAd5 HIV-1 gag/pol/nef trivalent vaccine based on a weakened non-replicating adenovirus (type 5 adenovirus). This was a Randomized, double blind, placebo control study carried out in North America, South America, Caribbean and Australia This trial enrolled 3000 adult HIV negative high-risk population from a diverse background (gay/MSM, female sex workers 18-45 years of age). The study had 62% males and 38% females. First enrolment was in Dec 2004 and the enrolment was completed in March 2007. The primary efficacy endpoints: Whether the vaccine prevented HIV infection and whether the vaccine reduced the viral load in those who were infected. Results: The vaccine did not prevent infection: in the 741 volunteers who received at least one dose of the three-dose vaccine series, 24 cases of HIV infection were observed and 21 cases of HIV ...