RV144 Trial: A Vaccine that triggered hope
A Phase III Trial of Sanofi Pasteur Live Recombinant ALVAC-HIV (vCP1521) Priming With VaxGen gp120 B/E (AIDSVAX B/E) Boosting in HIV-Uninfected Thai Adults.
RV114 was a Prevention, Randomized, Double Blind (Subject, Caregiver, Investigator), Placebo Control, Parallel Assignment, Efficacy Study carried out in Thailand.
This prime-boost test-of-concept trial began in 2003 and enrolled 15403 HIV-negative Thai men and women between the ages of 18 and 30. The vaccine consisted of 2 regimens: ALVAC (prime) and AIDSVAX B/E (boost). The vaccine consisted of synthetic fragments of genetic components of HIV- subtypes B and E (CRF01 AE).
End points: Whether vaccine reduced risk of HIV infection and whether vaccinated individuals who acquired HIV had lower viral loads than placebo group.
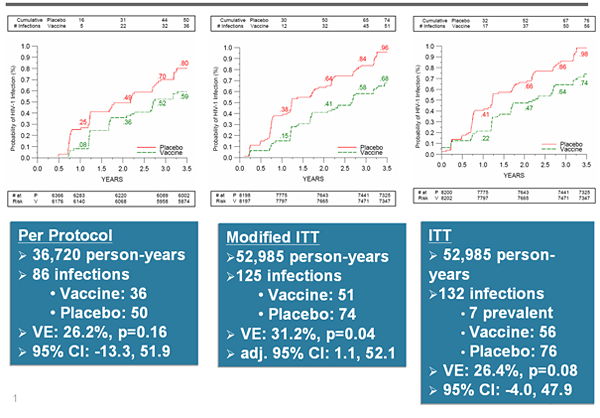
To determine statistical significance, data were analyzed according to 1. Intent to treat (ITT) 2. Modified Intent to treat (mITT) and 3. Per Protocol (PP).
Results:
* The vaccine lowered the rate of infection by 31.2 % (mITT analysis, n=51 vs. n=74, p=0.04)
* IgG antibodies that bind to V1/V2 of HIV envelope correlated with lower infection rates among vaccinees.
* Vaccine efficacy peaked early at 60.5% at 12 months (cumulative efficacy, 95% CI 22-80) and then declined.
* PP, mITT and ITT analyses showed same trend with fewer infections in treatment group compared to placebo, showing a reduction in HIV acquisition risk between 25.2% and 31.2%.
* HIV genome sequences from 110 RV144 participants who became HIV infected showed that antibodies directed at the V1V2 region reduced the risk of infection.
* The vaccine lowered the rate of infection by 31.2 % (mITT analysis, n=51 vs. n=74, p=0.04)
* IgG antibodies that bind to V1/V2 of HIV envelope correlated with lower infection rates among vaccinees.
* Vaccine efficacy peaked early at 60.5% at 12 months (cumulative efficacy, 95% CI 22-80) and then declined.
* PP, mITT and ITT analyses showed same trend with fewer infections in treatment group compared to placebo, showing a reduction in HIV acquisition risk between 25.2% and 31.2%.
* HIV genome sequences from 110 RV144 participants who became HIV infected showed that antibodies directed at the V1V2 region reduced the risk of infection.
* Vaccine regimen was safe and modestly effective in preventing HIV infection
Limitations:
1. The vaccine studied here is HIV subtype E, which is prevalent in Southeast Asia. There is no evidence that the vaccine would be effective in areas where other subtypes are prevalent.
2. An important point to consider is whether the statistical effect seen here is because of the large sample size and the low risk population included in this study. If a similar population size was included in the STEP trial (high risk group), then perhaps a statistical effect might have been noted.
Future:
RV305 a randomized, double-blinded, placebo trial is being conducted to define the relative contributions of ALVAC-HIV alone or AIDSVAX B/E alone or their combination. So far, it seems that residual antibody response against gp120 and both gp70V1V2 scaffolds were detected 6-8 years post complete vaccination in RV144, suggesting anamnestic responses induced by late booster vaccinations. IgG response analysis support the prime-boost concept and ALVAC-HIV priming. IgA, IgG1 and IgG3 responses to gp120 were similar only in ALVAC-HIV/AIDSVAX-B/E and AIDSVAX-B/E groups indicating that ALVAC-HIV alone does not substantially contribute to these responses (Karasavvas, 2013 e-poster)
Future:
RV305 a randomized, double-blinded, placebo trial is being conducted to define the relative contributions of ALVAC-HIV alone or AIDSVAX B/E alone or their combination. So far, it seems that residual antibody response against gp120 and both gp70V1V2 scaffolds were detected 6-8 years post complete vaccination in RV144, suggesting anamnestic responses induced by late booster vaccinations. IgG response analysis support the prime-boost concept and ALVAC-HIV priming. IgA, IgG1 and IgG3 responses to gp120 were similar only in ALVAC-HIV/AIDSVAX-B/E and AIDSVAX-B/E groups indicating that ALVAC-HIV alone does not substantially contribute to these responses (Karasavvas, 2013 e-poster)
Comments
Post a Comment