STEP Trial: A vaccine that surprised many with increased risk of disease in vaccinees
HVTN/STEP Trial: A vaccine that surprised many with
increased risk of disease in vaccinees: A Phase IIb ‘test of concept’ trial of
Merck’s MRKAd5 HIV-1 gag/pol/nef trivalent vaccine based on a weakened
non-replicating adenovirus (type 5 adenovirus).
This was a Randomized, double blind,
placebo control study carried out in North America, South America, Caribbean
and Australia
This trial enrolled 3000 adult HIV
negative high-risk population from a diverse background (gay/MSM, female sex
workers 18-45 years of age). The study had 62% males and 38% females. First
enrolment was in Dec 2004 and the enrolment was completed in March 2007. The
primary efficacy endpoints: Whether the vaccine prevented HIV infection and
whether the vaccine reduced the viral load in those who were infected.
Results:
The vaccine did not
prevent infection: in the 741 volunteers who received at least one dose of the
three-dose vaccine series, 24 cases of HIV infection were observed and 21 cases
of HIV infection were observed in the 762 participants in the placebo group.
In the subgroup who
had received at least two vaccinations and who were HIV-negative for at least
the first twelve weeks of the trial, 19 cases of HIV infection were observed in
the 672 volunteers who received vaccine and 11 cases were observed in the 691
volunteers who received placebo.
These were interim
figures: later on, when all figures were collected, it was found that there
were 49 infections in patients receiving the vaccine and 33 in those receiving
placebo. This 48% higher rate of infection in vaccine recipients was not
statistically significant and could have been a random result.
HIV RNA levels
approximately eight to twelve weeks after diagnosis of infection were similar
in the vaccine and the placebo arms. The geometric means of the HIV RNA levels
in the blood of infected individuals, the standard measure of ongoing HIV
replication, were approximately 40,000 copies/ml in the vaccine group and
approximately 37,000 copies/ml in the placebo group.
Post-hoc analysis
showed that the vaccine made some participants more vulnerable to HIV, but was
not statistically significant, except for a subgroup of males.
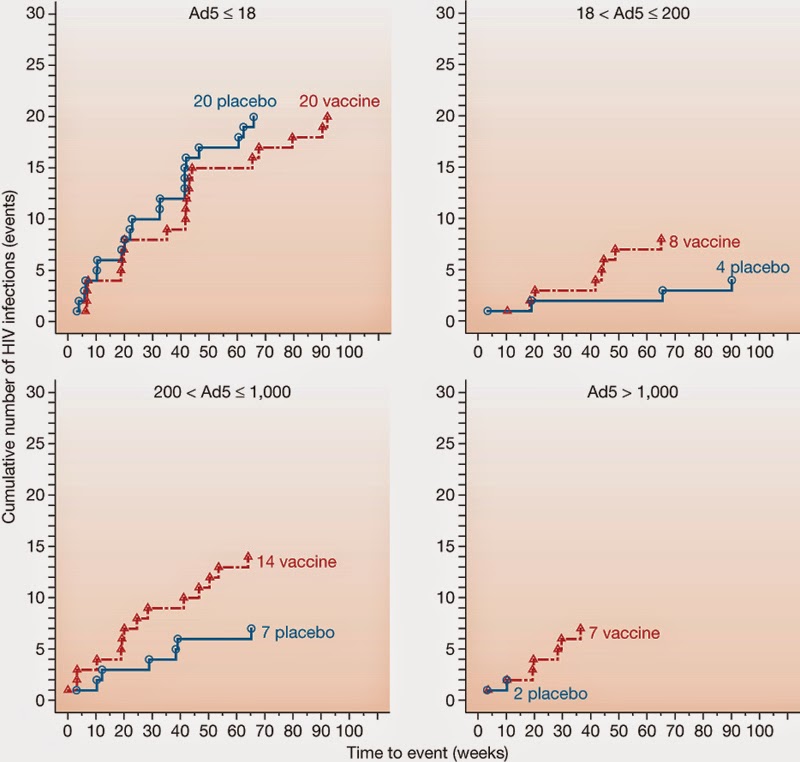
There was higher risk
of infection among those who had high levels of Ad5 immunity that was 3x higher
in vaccinees than in placebo group.
Uncircumcised
vaccinees were 4x higher infection rates than placebo, especially through
insertive anal sex.
1 female volunteer
was infected.
Weak HIV-specific CD8
T cell response directed against limited T cell epitopes, particularly HIV Gag.
Individuals with HLA –I (B27, b57 and B5801) had lower HIV RNA levels
Critical analysis of
this study revealed that having ineffective CD8 T cells will not help in a
better vaccine. The impact of having protective HLA alleles was shown on analysis
of participants who had better control of viral load.
Better vaccines could
be designed by deleting immunodominant epitopes restricted by non-protective
alleles from the immunogens since the induction of ineffective CD8 responses
will be magnified at the expense of effective CD8 response.
Comments
Post a Comment