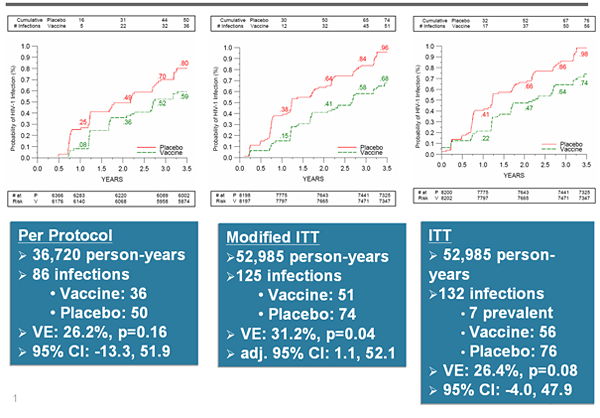
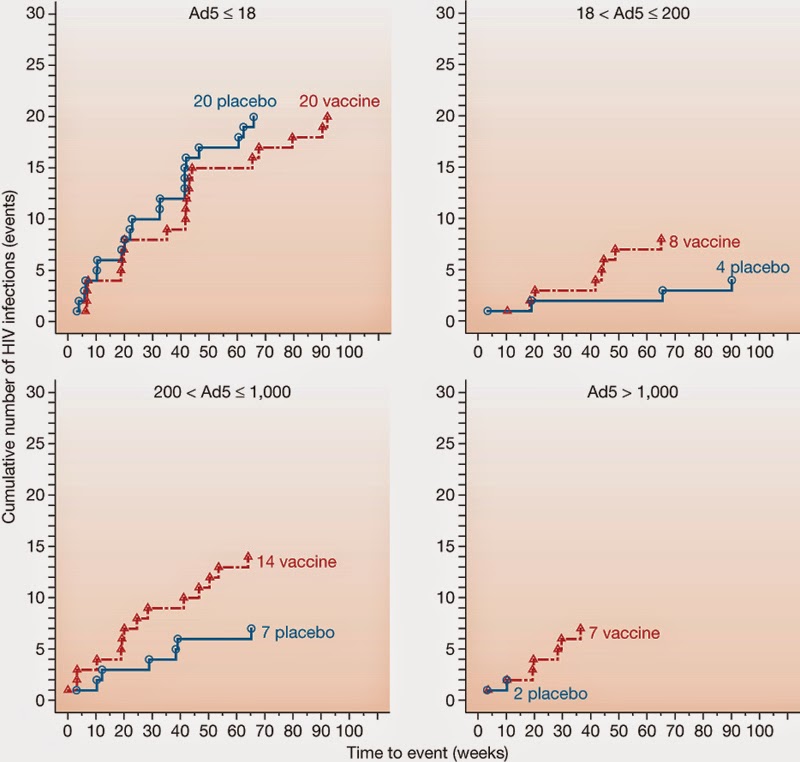
Chimp Adenovirus -based Ebola vaccine-Looks promising
A promising new study shows that a recombinant chimp adenovirus-3 vaccine vector carrying the surface glycoproteins of Zaire and Sudan strains of Ebola virus was safe and immunogenic in 20 volunteers. There were 11 women and 9 men in the study and the ages ranged from 25-50. Results 1. What looked really promising was that in about 70-90% of the participants, the vaccine induced antibodies to Zaire and Sudan glycoproteins. 2. Glycoprotein-specific memory CD4 and CD8 T cell responses were polyfunctional and the vaccine elicited high proportions of CD8 cells co-producing interferon-g and TNF. 3. Pre-existing antibodies against adenovirus-3 did not significantly correlated with the magnitude of vaccine-induced memory CD4 T cell response. Remarks * While the CD4 T cell shows no significant change in cytokine response with increased particle units, the polyfunctionality of CD8 T cells shows a significant change. * The graphs representing "Total Cytokine Response shown a